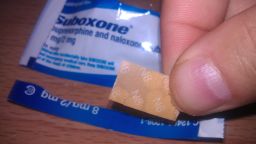
Reckitt Benckiser Group has agreed to pay up to $1.4 billion to resolve a long-running United States federal investigation into the company’s sales and marketing of Suboxone, a prescription drug used to treat people addicted to heroin, painkillers and other opioids.
The settlement is the largest in United States history in a case involving an opioid medication.
“Drug manufacturers marketing products to help opioid addicts are expected to do so honestly and responsibly,” Jody Hunt, assistant attorney general for the US Department of Justice’s Civil Division, said in a news release from the department on Thursday.
“We are confronting the deadliest drug crisis in our nation’s history. Opioid withdrawal is difficult, painful, and sometimes dangerous; people struggling to overcome addiction face challenges that can often seem insurmountable.”
Reckitt Benckiser announced on Thursday it had reached agreements with the US Department of Justice and the Federal Trade Commission, noting in a statement that the resolution will protect the group’s participation in US government programs. The statement said that the settlement amount will be funded through existing borrowing facilities and cash generation.
The statement also said that Reckitt Benckiser “has acted lawfully at all times and expressly denies all allegations that it engaged in any wrongful conduct.”
The civil settlement addresses allegations by the United States that, from 2010 through 2014, Reckitt Benckiser directly or through its subsidiaries mishandled the marketing or improperly controlled the pricing of Suboxone.
The US Food and Drug Administration approved the sale of Suboxone tablets, a mixture of buprenorphine and naloxone made by Indivior, in 2002.
Indivior, which was formerly part of Reckitt Benckiser, demerged from the group in 2014.
In April, Indivior was indicted “for engaging in an illicit nationwide scheme to increase prescriptions of Suboxone Film,” according to the Justice Department.
According to that indictment, Indivior developed Suboxone Film around 2007 as an alternative to Suboxone tablets, which were on the verge of facing generic drug competition at the time.
The indictment claims that Indivior obtained billions of dollars in revenue from the film version of Suboxone by “fraudulently marketing” it as safer and less abusable than the tablet form. The criminal trial against Indivior is scheduled to begin on May 11, 2020, in the US District Court in Abingdon, Virginia, according to the Justice Department.
Get CNN Health's weekly newsletter
Sign up here to get The Results Are In with Dr. Sanjay Gupta every Tuesday from the CNN Health team.
“Opioid addiction and abuse is an immense public health crisis and taking steps to address it is one the FDA’s highest priorities,” Dr. Ned Sharpless, acting commissioner of the FDA, said in the Justice Department’s news release on Thursday.
“Providing misleading information about product benefits puts the public at risk. We also are particularly concerned with schemes to game the drug approval process to prevent generic competition for important medicines,” he said. “The FDA, including criminal investigators in our Office of Regulatory Affairs and the lawyers in our Office of Chief Counsel, will continue to work with the Department of Justice to investigate and hold accountable those who devise and participate in schemes to the detriment of the public health.”