Story highlights
Scientists have cloned human stem cells
They have no intention of cloning humans
The technique could be used for mitochondrial diseases
A human embryo, containing about a couple hundred cells, is smaller than the period at the end of a sentence. Scientists need strong microscopes to see these precursors to life, and to take from them stem cells, which have the potential to become any cell in the body.
Earlier this week a breakthrough in this field was announced. A group of researchers published in the journal Cell proof that they had created embryonic stem cells through cloning. The scientists produced embryos using human skin cells, and then used the embryos to produce stem cell lines.
“It is an incredibly powerful approach with potential to generate almost any tissue in the body, genetically identical to the patient,” said Jeff Karp, associate professor at Harvard Medical School and co-director of the Center for Regenerative Therapeutics at the Brigham and Women’s Hospital in Boston.
Creating an embryo just from an egg and a skin cell seems like magic, but just how practical would the subsequent stem cells be? And does it actually amount to cloning?
What they did
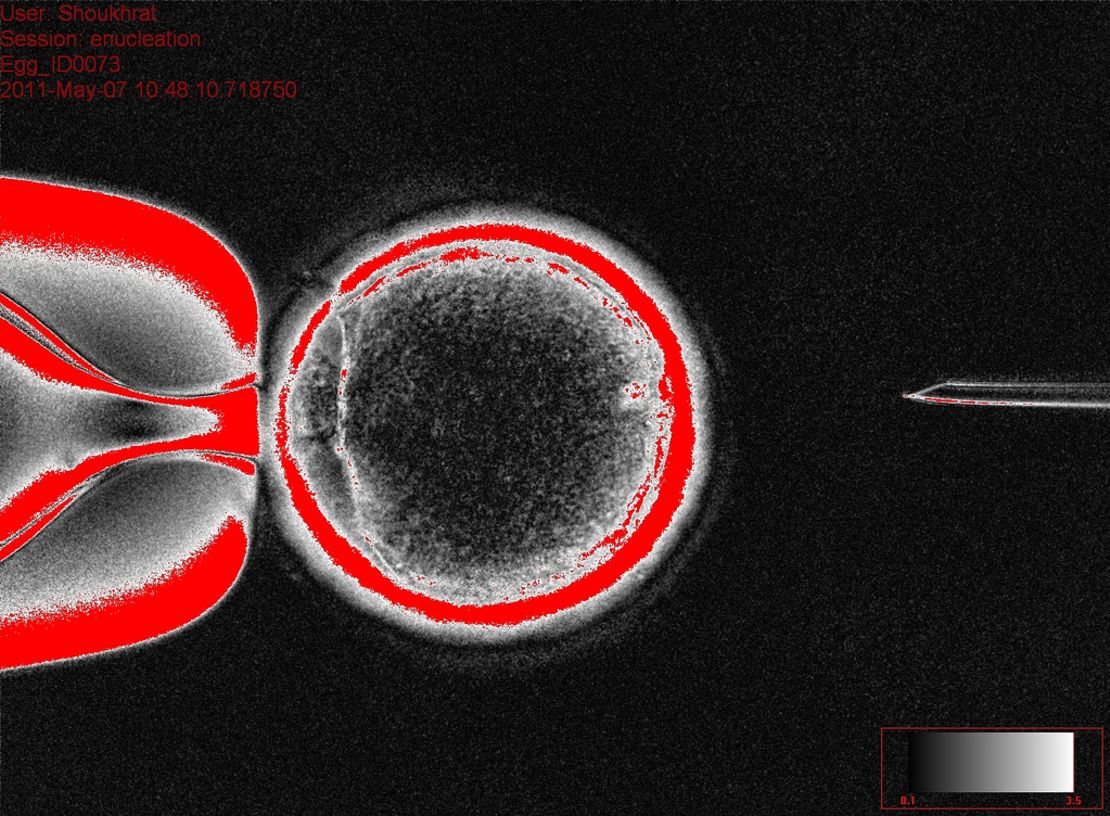
Normally, an embryo is created when sperm enters the egg and it starts to divide. But, in the Cell study, Shoukhrat Mitalipov and colleagues at Oregon Health & Science University began with skin cells from an 8-month-old baby that had a genetic disease. They did not use sperm.
To create each embryo, they took the DNA out of an egg, so that it was hollow, and replaced it with the skin cell’s DNA instead. The baby’s DNA was the only genetic material being used.
With the help of chemicals, the egg started to divide just like a normal fertilized egg would. Then, within several days, embryos genetically identical to the baby were created, from which stem cells were derived.
Embryonic stems research is inherently controversial because in order to use the stem cells for science, the embryo, which is a collection of cells that could develop into a fully formed human, is destroyed, even though embryos in these procedures are left over from in vitro fertilization.
However, Mitalipov said the embryos created in his study, from skin cells and eggs, would not grow babies. That would have required additional technology, and it wasn’t part of the study.
While cloning stem cells is a technical breakthrough, there’s already a method of deriving embryonic-like stem cells that doesn’t require the use of embryos at all: induced pluripotent stem (IPS) cells, said Dr. George Daley, who is director of the Stem Cell Transplantation Program at Children’s Hospital Boston and an international expert in stem cells.
Induced pluripotent stem cells can come from any cell in the human body, including skin cells, so they don’t have the moral quandaries surrounding them. Researchers have developed methods of inserting genes to “turn back the clock” on cells that have already specialized, so that they can turn into anything again. It doesn’t matter what the cell was before; it can now be reprogrammed as any kind of cell researchers want.
The new study involves a complex method that requires women to donate eggs, and a demanding manipulation of cell components on a tiny scale, Daley said.
What remains to be seen is whether these cloned embryonic stem cells are more useful therapeutically than the noncontroversial induced pluripotent stem cells, and questions linger about their effectiveness.
What’s the best type of stem cell
Ethical questions aside, researchers say they need to explore both embryonic and induced pluripotent stem cells in order to see what works best for various diseases and conditions.
Safety concerns linger around induced pluripotent stem cells because they were first created inserting four new genes.
“Remember, this was a genetic manipulation that was done to generate those cells, and there is concern that (for) anything you derive from them and you put back in the patient as graft, you may be at risk,” said John Gearhart, director of the Institute for Regenerative Medicine at the University of Pennsylvania, and one of the leading pioneers of stem cell research.
New techniques have been developed, however, to make induced pluripotent stem cells without permanent genetic modifications that were associated with tumors.
In mice, Daley and colleagues have shown that stem cells derived from the nuclear transfer of cells to make embryos – the technique described in Mitalipov’s paper – were indeed closer to natural embryo stem cells than induced pluripotent stem cells. The differences were so subtle that they may not be meaningful, however, he said.
The new study involves something similar to the cloning technique that led to the birth of Dolly, the famous cloned sheep that was born in July 1996. But making embryos for reproduction would require more advanced, complex techniques than were used in the new study – and serious scientists do not endorse human cloning for reproduction.
Mitalipov, senior author on the paper, laughs when asked if he wants to clone a person. “No, of course not,” he said.
“We tried the same approach to clone monkeys, because we’d been interested for biomedical research to produce cloned monkeys, and it never worked,” he said. “We’ve been working for a decade in that area.”
Mitalipov and colleagues had no intention of this research leading to the birth of a cloned human.
Researchers say there have been so many health problems in cloned animals, including Dolly herself, that it would not be ethical to attempt to create a cloned human.
“No legitimate scientist would be stepping forward to apply this in reproductive cloning, or for fertility work,” Daley said. “I would argue that really there are no good medical reasons to generate a cloned baby.”
So what is it good for?
There’s one important area where experts say Mitalipov’s method could have tremendous implications: Mitochondrial disease.
The mitochondria are the “power plants” of cells, supplying them with chemical energy. DNA in the mitochondria is inherited entirely from the mother’s egg, unlike the DNA in the cell’s nucleus, which comes from both parents.
Mutations in mitochondrial DNA can lead to deadly diseases, and their associated mutations are passed down to each new generation. Induced pluripotent stem cells preserve these harmful mutations, says Mitalipov.
A cell’s mitochondrial DNA develops mutations over the course of a lifetime, little by little, and may result in diseases such as Parkinson’s disease and diabetes, Mitalipov said. It’s possible, he says, that one day there will be stem cell treatments for aging and age-related diseases.
The only way to ensure that stem cells derived from an adult patient do not have mitochondrial DNA mutations would be to use the technique demonstrated in the new study, Mitalipov said: Creating embryos with cells from the patient’s own body, and healthy eggs, for the purpose of deriving embryonic stem cells.
“You want 0 miles in (the) rejuvenated cells that you want to put back into these patients,” he said. “The 0 mileage engine is in the egg.”
Mitalipov’s group also demonstrated in a 2012 Nature study that it could be possible to, using genetic techniques, reconstruct embryos that would not have the unhealthy mitochondrial mutations. This is not cloning, but draws on similar knowledge, and could cure a family’s genetic disease lineage in the future.
What’s next?
Daley estimates human clinical trials in stem cells will start within one to three years, but perhaps it could take a decade or more before the impact of stem cell therapy becomes widespread.
Gearhart is confident that the more we learn how to manipulate stem cells safely, there will be safe way to provide them to patients who need them. But there are different levels of risk for different uses. There will always be a risk-benefit calculation to be made, he said.
Different areas of stem cell research have proven to be harder than others. Beta cells for type I diabetes have been “a very difficult nut to crack,” Gearhart said, but there have been promising developments in repairing the heart, something that his lab has worked on, as well as for eye diseases.
“I think it’s going to be exciting times over the next several years when it comes to this,” he said.